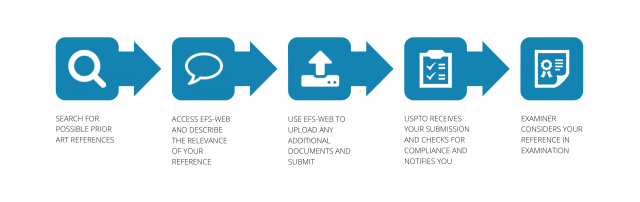
Protecting your intellectual property, whether domestically or internationally, is traditionally a complex and costly process. On an international scale, that cost is largely due to translations, with the largest patent filers often budgeting millions of dollars each year to ensure accurate translations are localized for each country in which they plan to file.

Legal teams might hire freelance translators with limited expertise to cut costs or partner with specialized patent agents in each country, especially if they have multiple patents to file in even more countries. The downside to this model is that while these foreign agents are familiar with the designated language, country and culture, there is often no communication between them and the inventor or with translators in other target countries. In addition, freelancers and local agents often lack sufficient scientific knowledge relevant to the patent. As a result, an error found and corrected by an agent in one country can easily be perpetuated as an error elsewhere. A single patent translation services provider, on the other hand, allows for streamlined management of the translation process that enables collaboration between the translator and agents for each country, and with the client.
Working with a single translation provider also allows inventors to often file more patent applications for the same amount of money that they would spend if they were to work with multiple providers. The advantage is that a single provider is able to leverage its capabilities across multiple jurisdictions, while reducing translation-related office actions and overall risks associated with multiple foreign agents and their independent translators.
“An effective IP translation service provider can leverage its resources and best practices in more jurisdictions than possible with one foreign agent, in part enabling patent rights in more geographic cases for the same cost – or even significantly lower,” said Ryan Marshall, a patent attorney and managing shareholder of Brinks Gilson & Lione’s Salt Lake City, Utah, office.
What are the “best practices” a company should consider to ensure quality translations that will help them increase patent filings, accelerate time to grant, reduce office actions and reduce the overall risk of patent?
- People: Patent translations require a fusion of both linguistic and industry-specific expertise. Optimal translation teams should consist of in-country native linguists, scientists, engineers and legal specialists.
- Process: Work with a patent translation service provider that uses a streamlined process with centralized project management, particularly if you are validating a patent in several countries and multiple languages. Working with a single project manager will create transparency, efficiency and accuracy in the translation and patent filing process.
- Technology: Translation technology should bolster the efforts of human translation teams by leveraging previous translations, maintaining a terminology database and simultaneously tracking the status of each patent across all languages and countries. This increases consistency and on-time delivery at a fair value.
By using a patent translation service provider that consolidated work to interactive and specialized translation teams reporting to a single project owner, Brinks’ client was able to validate a patent in 15 European countries, five more than originally planned. According to Marshall, the validation process was much more consistent across all jurisdictions and much less costly than if isolated foreign agents worked on the project in multiple jurisdictions.
The Importance of Trust
Translation is all about trust, as few companies know where inaccurate translations exist until they receive an office action or are in court facing the invalidation of the patent due to a mistranslated or misinterpreted word. Ensuring any service provider you work with (especially one providing the translations of your critical IP) follows best practices can instill the confidence that you’ll receive timely and quality translations, speeding up time to grant and reducing the total overall cost of patent ownership.
Today’s Guest Post is by Guest Barista Michael Degn, vice president of global sales at MutliLing, the innovative leader in intellectual property (IP) translations and related support services for foreign patent filings. He is a native English speaker but also fluent in Spanish.