In Takeda v. Alphapharm and Genpharm (06-1329), The U.S. Court of Appeals for the Federal Circuit upheld an earlier decision in the district court finding that U.S. Patent 4,687,777 was not invalid under 35 U.S.C. § 103. Takeda Chem. Indus., Ltd. v. Mylan Labs., 417 F. Supp. 2d 341 (S.D.N.Y. 2006).
Earlier, Takeda invented certain thiazolidinediones (TZDs), which were then found to be useful for the treatment for Type 2 diabetes. The TZDs acted as insulin sensitizers, i.e., compounds that ameliorate insulin resistance. Although the function of TZDs was not completely understood, TZDs appeared to lower blood glucose levels by binding to a molecule in the nucleus of the cell known as PPAR-gamma, which activates insulin receptors and stimulates the production of glucose transporters. The transporters then travel to the cellular surface and enable glucose to enter the cell from the bloodstream.
Takeda developed the drug ACTOS®, which is used to control blood sugar in patients who suffer from Type 2 diabetes, and had gross sales of over $1.7 billion in 2003. The active ingredient in ACTOS® is the TZD compound pioglitazone, a compound claimed in the ‘777 patent.
The ‘777 patent is directed to compounds which can be used as antidiabetic agents. Claim 1 claims a genus of compounds. Claim 5 claims pharmaceutical compositions containing that genus of compounds.
1. A compound of the formula:
or a pharmacologically acceptable salt thereof.
5. An antidiabetic composition which consists essentially of a compound of the formula:
or a pharmacologically acceptable salt thereof, in association with a pharmacologically acceptable carrier, excipient or diluent.
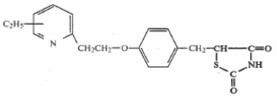
The critical portion of the compound structure is the left moiety of the molecule, namely, the ethyl-substituted pyridyl ring. That chemical structure, which has an ethyl substituent (C2H5) pictorially drawn to the center of the pyridyl ring, indicates that the structure covers four possible compounds, that is, compounds with an ethyl substituent located at the four available positions on the pyridyl ring. The formula includes the 3-ethyl compound, 4-ethyl compound, 5-ethyl compound (pioglitazone), and 6-ethyl compound.
Claim 2 of the ’777 patent covers the single compound pioglitazone. That claim, which depends from claim 1, reads:
2. A compound as claimed in claim 1, wherein the compound is 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione.
Pioglitazone is referred to as the 5-ethyl compound because the ethyl substituent is attached to the 5-position on the pyridyl ring. That portion of the compound is depicted as:
Alphapharm, a generic drug manufacturer, filed an Abbreviated New Drug Application (ANDA) pursuant to the Hatch-Waxman Act seeking FDA approval under 21 U.S.C. § 355(j) et seq. to manufacture and sell a generic version of pioglitazone. Alphapharm filed a Paragraph IV certification with its ANDA pursuant to § 505(j)(2)(B)(ii), asserting that the ‘777 patent is invalid as obvious under 35 U.S.C. § 103.
So, as these things go, Takeda sued Alphapharm, along with three other generic drug manufacturers who also sought FDA approval to market generic pioglitazone, alleging that the defendants have infringed or will infringe the ‘777 patent.
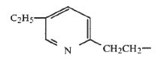
On January 17, 2006, the district court commenced a bench trial solely on the issues of validity and enforceability of the ‘777 patent. Alphapharm advanced its invalidity argument, asserting that the claimed compounds would have been obvious at the time of the alleged invention. Alphapharm’s obviousness contention rested entirely on a prior art TZD compound that is referenced in Table 1 of the ‘777 patent as compound b. The left moiety of compound b consists of a pyridyl ring with a methyl (CH3) group attached to the 6-position of the ring. That portion of its chemical structure is illustrated as follows:
Alphapharm asserted that the claimed compounds would have been obvious over compound b.
The district court found that Alphapharm failed to prove by clear and convincing evidence that the asserted claims were invalid as obvious under 35 U.S.C. § 103. The court first concluded that there was no motivation in the prior art to select compound b as the lead compound for antidiabetic research, and that the prior art taught away from its use. As such, the court concluded that Alphapharm failed to make a prima facie case of obviousness. The court also found that even if Alphapharm succeeded in making a prima facie showing, Takeda would still prevail because any prima facie case of obviousness was rebutted by the unexpected results of pioglitazone’s nontoxicity.
On appeal, Alphapharm argued that the claims would have been obvious.
First, Alphapharm argued that the district court misapplied the law, particularly the law governing obviousness in the context of structurally similar chemical compounds. According to Alphapharm, compound b was the most effective antidiabetic compound in the prior art, and thus the court erred by failing to apply a presumption that one of ordinary skill in the art would have been motivated to make the claimed compounds.
Second, Alphapharm argued that the court erred in determining the scope and content of the prior art, in particular, whether to include the prosecution history of the prior ‘779 patent.
The Federal Circuit sided with Takeda citing KSR International Co. v. Teleflex Inc., 127 S. Ct. 1727 (2007). The Court stated that the Graham v. John Deere Co. of Kansas City, 383 U.S. 1 (1966), factors still control an obviousness inquiry. Those factors are: 1) “the scope and content of the prior art”; 2) the “differences between the prior art and the claims”; 3) “the level of ordinary skill in the pertinent art”; and 4) objective evidence of nonobviousness.
The court held that:
Our case law concerning prima facie obviousness of structurally similar compounds is well-established. We have held that “structural similarity between claimed and prior art subject matter, proved by combining references or otherwise, where the prior art gives reason or motivation to make the claimed compositions, creates a prima facie case of obviousness.” Dillon, 919 F.2d at 692. In addition to structural similarity between the compounds, a prima facie case of obviousness also requires a showing of “adequate support in the prior art” for the change in structure. In re Grabiak, 769 F.2d 729, 731-32 (Fed. Cir. 1985).
A known compound may suggest its homolog, analog, or isomer because such compounds “often have similar properties and therefore chemists of ordinary skill would ordinarily contemplate making them to try to obtain compounds with improved properties.” We clarified, however, that in order to find a prima facie case of unpatentability in such instances, a showing that the “prior art would have suggested making the specific molecular modifications necessary to achieve the claimed invention” was also required. Id. (citing In re Jones, 958 F.2d 347 (Fed. Cir. 1992); Dillon, 919 F.2d 688; Grabiak, 769 F.2d 729; In re Lalu, 747 F.2d 703 (Fed. Cir. 1984)).
That test for prima facie obviousness for chemical compounds is consistent with the legal principles enunciated in KSR. While the KSR Court rejected a rigid application of the teaching, suggestion, or motivation (TSM) test in an obviousness inquiry, the Court acknowledged the importance of identifying “a reason that would have prompted a person of ordinary skill in the relevant field to combine the elements in the way the claimed new invention does” in an obviousness determination. KSR, 127 S. Ct. at 1731. Moreover, the Court indicated that there is “no necessary inconsistency between the idea underlying the TSM test and the Graham analysis.” As long as the test is not applied as a “rigid and mandatory” formula, that test can provide “helpful insight” to an obviousness inquiry. Thus, in cases involving new chemical compounds, it remains necessary to identify some reason that would have led a chemist to modify a known compound in a particular manner to establish prima facie obviousness of a new claimed compound.



[…] Patent Baristas has a terrific post on the Takeda case. Filed under: Patent Permalink | Trackback URL| […]